Cryovial Filler
Pharma Equipment
- pharma equipment
- DISCOVER - R&D scale
- Cryovial Filler
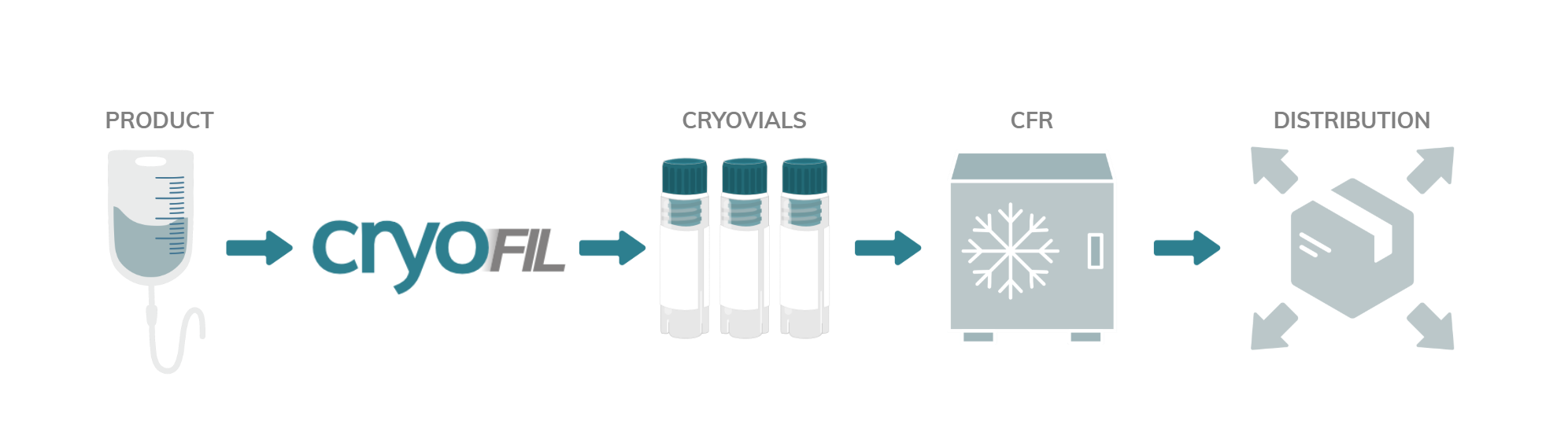
cryoFIL® - cGMP cryovial filling platform
cryoFIL® enhances cell viability and maximises yield through automated cryovial filling and handling.
In the rapidly growing Cell & Gene Therapy (CGT) sector, many processes remain manual, raising contamination risks from operator handling. The cryoFIL® system automates your cryovial filling process and uses Controlled Rate Freezer (CRF) compatible racks, providing seamless integration.
From small to large batch sizes - whether 10 or 1000 vials - we comply with current regulatory standards to offer a scalable solution for your cell therapy needs.
How does cryoFIL® work?
Cryovials are transferred from the rack to the filling module. Each vial is individually uncapped, filled and recapped before being returned to its original position in the rack.
The rack can then be placed directly into the freezer, eliminating the need for further handling of the vials. This process maintains the integrity of your samples and reduces the risk of contamination.
CS Banking proccess




Get in touch
Sterility assurance
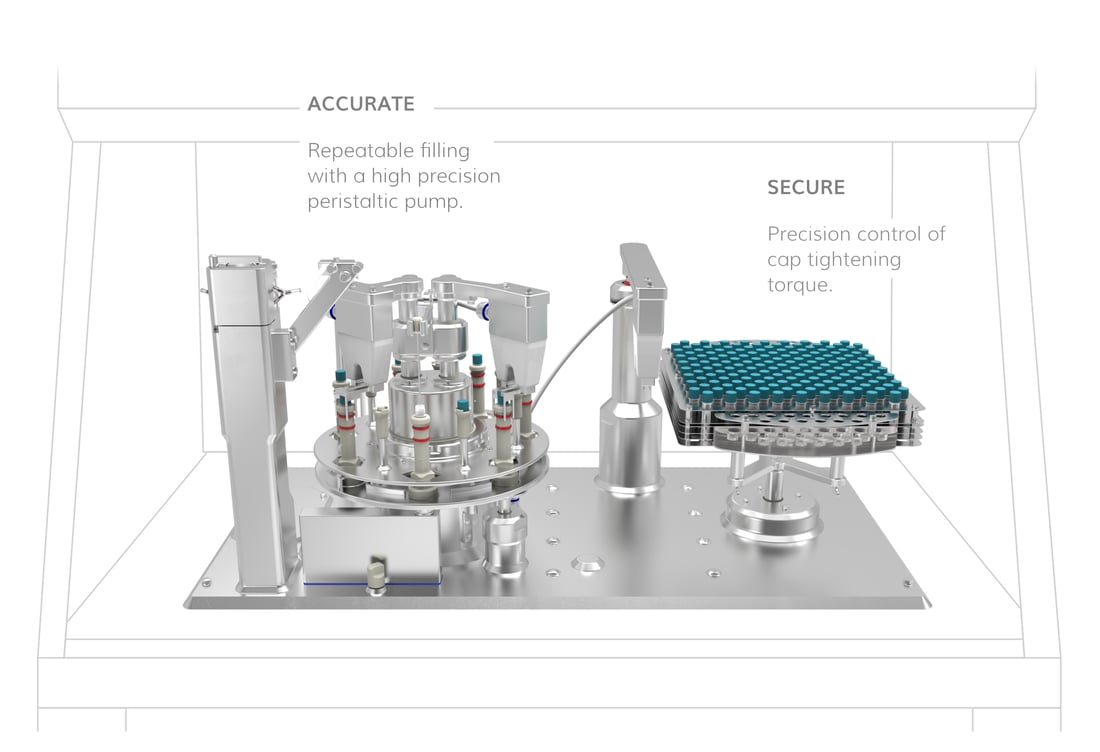
Our advanced aseptic design ensures that vials are opened for the shortest possible duration, and only exposed to 'first air', significantly reducing the risk of contamination.
Multi-format flexibility
Process multiple cryovial types seamlessly on the same platform, thanks to a modular design with quick swap change parts. Onboard recipe settings allow for rapid format adjustments, reducing downtime and enhancing operational efficiency. This versatility makes it easy to scale production while accommodating evolving process requirements.
Maximises cell yield
Maximise the number of in-spec fills with intelligent closed-loop filling, minimised hold-up volume, and a zero-loss priming sequence. Real-time 100% weight verification ensures precise dosing, helping to preserve valuable cell therapies.
Touch screen interface
A user-friendly, password-protected touchscreen interface provides intuitive control with multi-level user access. The system generates 21 CFR part 11 compliant batch reports.
Ongoing support
Installation is just the beginning. Our dedicated customer care team provides ongoing technical support, preventative maintenance, and optimisation services to ensure the long-term reliability and performance of your machine. From training to spare parts and remote diagnostics, we’re committed to keeping your operations running smoothly. For more information, head over to our customer care page.
Technical details
- Compatible with 0.5-5ml cryovials from all major brands.
- Up to 5ml fills with typical accuracy of +/-1%.
- Integration into BSC as standard, isolator options available
- Up to 600 cycles/hour.
-
Lorem ipsum dolor amet aesthetic photo booth activated charcoal occupy iPhone schlitz squid. Everyday carry 3 wolf moon raw denim semiotics pok pok tattooed readymade bushwick. Humblebrag skateboard green juice mixtape polaroid ethical, messenger bag pitchfork sriracha hammock. Fam twee 3 wolf moon, authentic woke stumptown bespoke.
-
Lorem ipsum dolor amet aesthetic photo booth activated charcoal occupy iPhone schlitz squid. Everyday carry 3 wolf moon raw denim semiotics pok pok tattooed readymade bushwick. Humblebrag skateboard green juice mixtape polaroid ethical, messenger bag pitchfork sriracha hammock. Fam twee 3 wolf moon, authentic woke stumptown bespoke.
-
Lorem ipsum dolor amet aesthetic photo booth activated charcoal occupy iPhone schlitz squid. Everyday carry 3 wolf moon raw denim semiotics pok pok tattooed readymade bushwick. Humblebrag skateboard green juice mixtape polaroid ethical, messenger bag pitchfork sriracha hammock. Fam twee 3 wolf moon, authentic woke stumptown bespoke.